Our Breakthrough

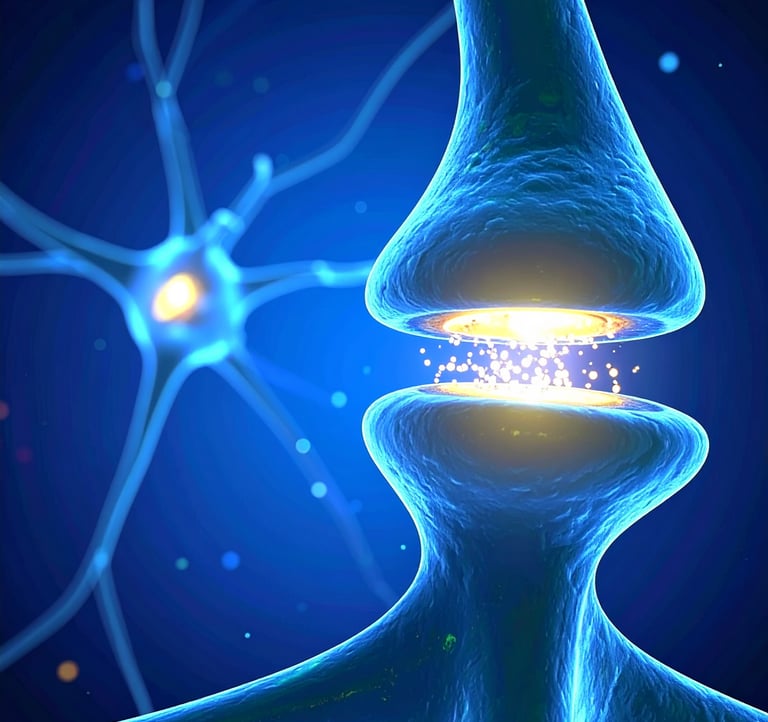
NeurANO’s hybrid nanotherapeutics NexiM and NexiK bypass the synapse to restore balance to the brain.
Unique Mechanism
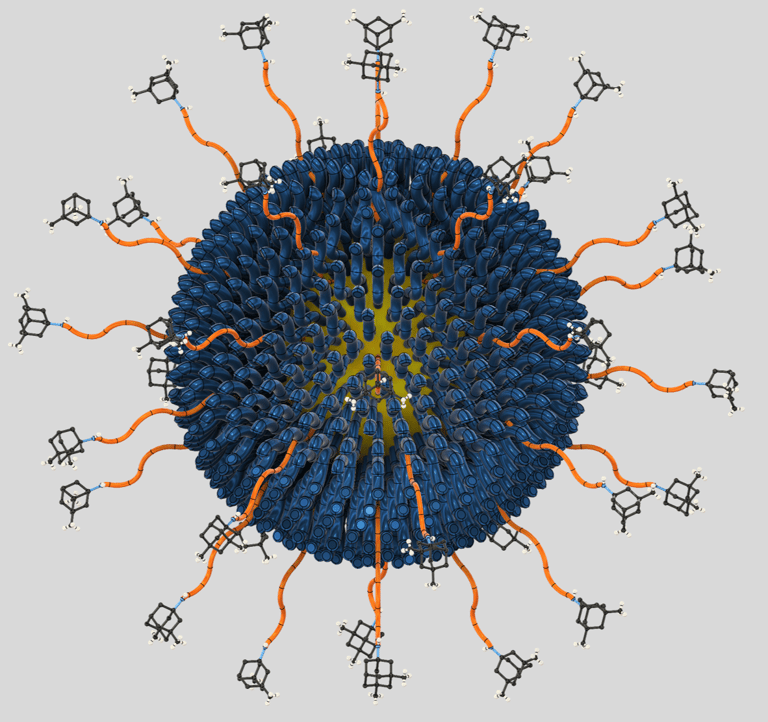
Our small molecule conjugate is too large to enter synaptic clefts and small enough to exclusively block extrasynaptic NMDARs implicated in disease.
Pathology-Specific
NexiM and NexiK modulate “bad” receptors without interfering with normal neurotransmission.
Rationally engineered from FDA-approved components for superior therapeutic index, with validated safety and efficacy in early small mammal trials.
Precise, Safe & Durable

Publications
Our founding scientists have been cited tens of thousands of times across publications including Nature, Journal of Neuroscience, Drug Discovery Today, Nano, and many more.
Read about some of our foundational publications below.


Oligomerized amyloid-β drives neurodegeneration in Alzheimer’s by overstimulating extrasynaptic NMDA receptors (eNMDARs), which trigger excessive nitric-oxide signaling and toxic protein modifications.
Our work identifies eNMDARs as the key mediator of Aβ-induced synaptic damage, highlighting a precise therapeutic target to reduce nitrosative stress and preserve neuronal function.


We developed AuM, a first-in-class hybrid nanodrug that selectively blocks extrasynaptic NMDA receptors (eNMDARs)—the pathological drivers of neurodegeneration—while preserving normal synaptic signaling. By coupling memantine to gold nanoparticles, AuM achieves targeted neuroprotection against excitotoxicity and amyloid-induced damage, offering a novel, spatially precise strategy for treating Alzheimer’s and other neurological disorders.
Non-genetic neuromodulation with graphene optoelectronic actuators for disease models, stem cell maturation, and biohybrid robotics
Nanostructured Antagonist of Extrasynaptic NMDA Receptors
Nano Letters volume 16(9): 5495-502.
Differential Effects of Synaptic and Extrasynaptic NMDA Receptors on Aβ-Induced Nitric Oxide Production in Cerebrocortical Neurons
Journal of Neuroscience volume 34 (14): 5023-5028
The Graphene-Mediated Optical Stimulation (GraMOS) platform uses graphene’s light-to-electric conversion to precisely and non-invasively control neuronal activity—without genetic modification. By enhancing neural maturation, revealing disease phenotypes, and even driving robotic control from brain organoids, GraMOS establishes a breakthrough interface for regenerative medicine, disease modeling, and bio-integrated robotics.
Address
9500 Gilman Blvd MS#0423
La Jolla CA 92093
Contact
business@neuranobio.com
Copyright © 2025
All rights reserved
